Ethynyl hydrogen bonds and iodoethynyl halogen bonds: a case of synthon mimicry†
Christer B.
Aakeröy
*,
Dhanushi
Welideniya
and
John
Desper
Department of Chemistry, Kansas State University, Manhattan, KS 66506, USA. E-mail: aakeroy@ksu.edu
First published on 25th October 2016
Abstract
The common electrostatic features of ethynyl and iodoethynyl hydrogen- and halogen-bond donors, respectively, lead to synthon mimicry which can be employed in synthetic crystal engineering for the construction of identical supramolecular assemblies in the solid-state.
Unlike in molecular chemistry where covalent bond-making/bond-breaking reactions have been mapped out through hundreds of named reactions, crystal structures (and physical properties in particular) are not easily related to molecular structure.1 However, by carefully examining the structural behavior and packing preferences of specific functional groups and their synthons, we may be able to make inferences about physical properties simply based upon molecular architecture.2 The connection is implicit because each structure arises from a careful balance between a multitude of recognition events among molecular functional groups. Hydrogen bonds3 (HBs) and halogen bonds (XBs)4 are arguably the most useful supramolecular synthetic tools due to their strength, selectivity, and directionality.5 These interactions are also to a considerable extent (although by no means exclusively6) driven by electrostatic complementarity between the donor-acceptor sites in each system.7 The most effective halogen-bond donors include an iodine atom thanks to its polarizability, especially if it is ‘activated’ by electron-withdrawing groups8 or if bound to an sp-hybridized carbon atom.9 This also suggests that hydrogen-bond and halogen-bond donors of the same geometry could give rise to identical architectures in the solid state despite the fact that the iodine atom is substantially larger than the hydrogen atom and they display drastically different electronic structures. Furthermore, if pairs of XB and HB donors produce similar structural outcomes, we would have further indication that the electrostatic component, inherent in both HBs and HBs, is very important to practical solid state assembly. Previously, the isostructurality of C–X (where X = Cl, Br, or I) and C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H groups has been amply demonstrated in single-component molecular solids,10 and the ethynyl group has also been denoted as a ‘supramolecular halogen’.11 However, in co-crystal synthesis of multi-component solids, little is known about whether ethynyl and haloethynyl groups exercise similar or different effects on the solid-state supramolecular assembly. The goal of this study is therefore to establish if ethynyl moieties capped by two atoms (hydrogen and iodine) of very different size and chemical characteristics nevertheless can display “synthon mimicry” in the context of practical co-crystal synthesis.
C–H groups has been amply demonstrated in single-component molecular solids,10 and the ethynyl group has also been denoted as a ‘supramolecular halogen’.11 However, in co-crystal synthesis of multi-component solids, little is known about whether ethynyl and haloethynyl groups exercise similar or different effects on the solid-state supramolecular assembly. The goal of this study is therefore to establish if ethynyl moieties capped by two atoms (hydrogen and iodine) of very different size and chemical characteristics nevertheless can display “synthon mimicry” in the context of practical co-crystal synthesis.
An effective way of probing structural consequences of intermolecular interactions is through the use of systematic co-crystallizations and in this work we employed 2-aminopyrimidine as a source of a reliable supramolecular structural backbone thanks to its propensity to form ribbon-like architectures via self-complementary NH⋯N hydrogen bonds.12 Thus we used two 2-aminopyrimidine based donor molecules, Ipym and Hpym (Scheme 1), to examine if C–I⋯N and C–H⋯N interactions can be used interchangeably as synthetic vectors for bridging the expected ribbons in the desired manner with the help of a suitable halogen/hydrogen bond acceptor.
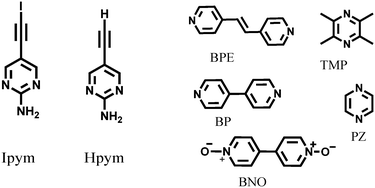 | ||
| Scheme 1 The donors (Ipym and Hpym) and five symmetric ditopic acceptors (BPE, TMP, BP, PZ and BNO). | ||
Hpym was synthesized through a Sonogashira coupling reaction followed by deprotection. Subsequent iodination of Hpym resulted in Ipym in good yield (80%). Hpym and Ipym were then allowed to react with tetramethyl pyrazine, 1,2-bis(4-pyridyl)ethylene, 4,4′-bipyridyl N,N′-dioxide, pyrazine, and 4,4′-bipyridine (Scheme 1) using solvent assisted grinding. Slow evaporation of the subsequent solution experiments (see ESI†) resulted in crystals suitable for single crystal X-ray diffraction of IPym·TMP, HPym·TMP, IPym·BPE and HPym·BPE, as well as for IPym and HPym by themselves.
In order to determine the magnitude of the electrostatic potential surrounding the donor atoms in Hpym and Ipym, respectively, we employed DFT calculations which showed that the hydrogen and the iodine atoms have comparable molecular electrostatic potentials, Fig. 1.
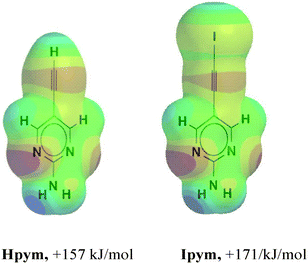 | ||
| Fig. 1 Maximum values on the molecular electrostatic surface of the ethynyl and the iodoethynyl species in Hpym and Ipym. | ||
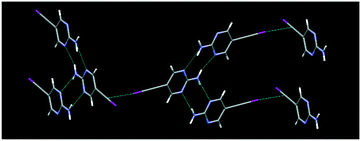
The structure determinations of Ipym and Hpym reveal that in both structures, the 2-aminopyrimidine moiety forms the expected and intended ribbon like arrangement via NH⋯N interactions. These ribbons further extend to a 3-D network via perpendicular C(sp)–I⋯π interactions in Ipym, Fig. 2, and a 2-D assembly via C(sp)–H⋯N interactions in Hpym, Fig. 3.
The structure determination of IPym·TMP showed a co-crystal in which the primary motif is constructed through self-complementary NH⋯N synthons from the 2-aminopyrimidine moiety to form a ribbon-like architecture. These ribbons are further cross-linked by C(sp)–I⋯N halogen bonds, Fig. 4. Similarly HPym·TMP resulted in a co-crystal where infinite ribbons formed via NH⋯N hydrogen bonds are interconnected via C(sp)–H⋯N hydrogen bonds. Overall both IPym·TMP and HPym·TMP exhibit same structural behaviour indicating that C(sp)–H⋯N hydrogen bonds can act as C(sp)–I⋯N synthon mimics, Fig. 4.
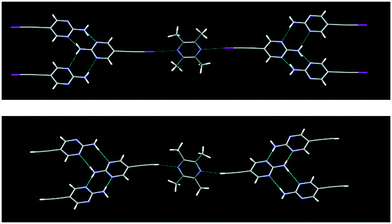 | ||
| Fig. 4 C(sp)–I⋯N halogen bonding (top) and C(sp)–H⋯N hydrogen bonding (bottom) play the same structural roles with TMP as the acceptor. | ||
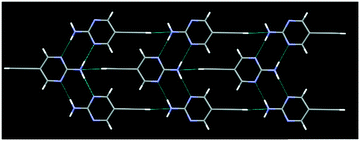
The crystal structures IPym·BPE and HPym·BPE also showed similar structural behaviour, where the symmetry related self-complementary NH⋯N synthon produces a ribbon like architecture. The C(sp)–I⋯N halogen bonds and C(sp)–H⋯N hydrogen bonds crosslink the pyrimidine molecules in both cases into infinite chains which again points to the synthon mimicry of C(sp)–I⋯N halogen bonds and C(sp)–H⋯N hydrogen bonds (Fig. 5).
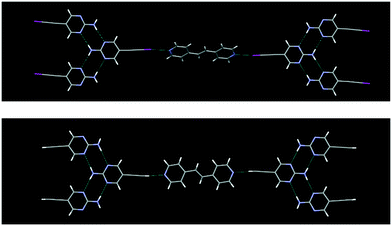 | ||
| Fig. 5 Despite changing the heterocyclic acceptor molecule (BPE), C(sp)–I⋯N halogen bonding (top) and C(sp)–H⋯N hydrogen bonding (bottom) display synthon mimicry. | ||
The C(sp)–I⋯N distances of 2.897(2) Å and 2.7859(17) Å in the two co-crystals of IPym are considerably shorter than the sum of the van der Waal radii for nitrogen (1.55 Å) and iodine (1.98 Å) atoms. The C(sp)–H⋯N distances of 2.414(16) in HPym·TMP and 2.341(18), 2.277(18) in HPym·BPE are also shorter than the sum of van der Waal radii for nitrogen (1.55 Å) and hydrogen (1.20 Å). All C(sp)–I⋯N and C(sp)–H⋯N interactions in these co-crystals display bond angles close to linear, again emphasizing the electrostatic nature of these contacts, Table 1.
| Structure | C–I⋯N (Å) | C–I⋯N (°) |
|---|---|---|
| IPym·TMP | 2.897(2) | 176.76(11) |
| IPym·BPE | 2.7859(17) | 175.11(7) |
| C–H⋯N (Å) | C–H⋯N (°) | |
| HPym·TMP | 2.414(16) | 166.2(14) |
| HPym·BPE | 2.341(18) | 169.0(15) |
| 2.277(18) | 170.5(15) |
Given the structural similarities between the IPym and Hpym pairs examined herein, it would be tempting to try to make molecular ‘alloys’ where the solid-state composition is controlled by reaction stoichiometry. Unfortunately, it was not possible to prepare such materials by melting due to the fact that one component in each pair decomposed upon heating. Our attempts at making alloys from solution were also unsuccessful due to significant differences in solubility between the compounds in each pair. Despite these observations, the findings presented herein indicate that ethynyl and iodoethynyl moieties can be used to generate identical solid-state assemblies via near linear and short C–H⋯N and C–I⋯N synthons, respectively. The electrostatic similarities between the two donors (as shown by their molecular electrostatic potential surfaces) provide a rational for why they can be employed as isostructural synthons for practical crystal engineering despite the many differences between hydrogen- and iodine atoms. The implications of this synthon mimicry are likely to be extended to solution-phase phenomena13 (including biologically relevant systems), and we are currently exploring the generality of our findings.
Conclusions
To most intents and purposes, the hydrogen atom and the iodine atom have very different physical and chemical characteristics, however, hydrogen bonding and halogen bonding share a common electrostatic foundation which heavily influences non-covalent interaction geometries. This means that the synthon mimicry of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–I⋯N halogen bonds and C
C–I⋯N halogen bonds and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯N hydrogen bonds can lead to near-identical molecular recognition events in solution as well as indistinguishable solid-state structural effects.
C–H⋯N hydrogen bonds can lead to near-identical molecular recognition events in solution as well as indistinguishable solid-state structural effects.
Acknowledgements
This material is based upon work supported by, or in part by, the U. S. Army Research Laboratory and the U. S. Army Research Office under contract/grant number W911NF-13-1-0387.Notes and references
- A. Dey, R. K. J. Jetti, R. Boese and G. R. Desiraju, CrystEngComm, 2003, 5, 248–252 RSC
.
- C. B. Aakeröy, T. K. Wijethunga, J. Benton and J. Desper, Chem. Commun., 2015, 51, 2425–2428 RSC
; C. B. Aakeröy, S. Forbes and J. Desper, J. Am. Chem. Soc., 2009, 131, 17048 CrossRef PubMed
.
- L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem., Int. Ed., 2001, 40, 2382–2426 CrossRef CAS
; G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565–573 CrossRef PubMed
; E. A. Archer, H. Gong and M. J. Krische, Tetrahedron, 2001, 57, 1139–1159 CrossRef
; C. B. Aakeröy and K. R. Seddon, Chem. Soc. Rev., 1993, 22, 397–407 RSC
; C. B. Aakeröy, J. Desper and M. M. Smith, Chem. Commun., 2007, 3936 RSC
.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386–395 CrossRef CAS PubMed
; P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2010, 12, 7748–7757 RSC
; K. Rissanen, CrystEngComm, 2008, 10, 1107 RSC
.
- A. C. Legon, Angew. Chem., Int. Ed., 1999, 38, 2686–2714 CrossRef CAS
; A. C. Legon, Phys. Chem. Chem. Phys., 2010, 12, 7736–7747 RSC
.
- S. J. Grabowski, Phys. Chem. Chem. Phys., 2013, 15, 7249–7259 RSC
; K. E. Riley and P. Hobza, Phys. Chem. Chem. Phys., 2013, 15, 17742 RSC
; P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2013, 15, 11178 RSC
; J. Hoja, A. F. Sax and K. Szalewicz, Chem. – Eur. J., 2014, 20, 2292 CrossRef CAS PubMed
.
- N. Mohan and C. H. Suresh, J. Phys. Chem. A, 2014, 118, 1697–1705 CrossRef CAS PubMed
.
- G. Valerio, G. Raos, S. V. Meille, P. Metrangolo and G. Resnati, J. Phys. Chem. A, 2000, 104, 1617–1620 CrossRef CAS
.
- J.-W. Zou, Y.-J. Jiang, M. Guo, G.-X. Hu, B. Zhang, H.-C. Liu and Q.-S. Yu, Chem. – Eur. J., 2005, 11, 740–751 CrossRef CAS PubMed
; K. Bouchmella, B. Boury, S. G. Dutremez and A. van der Lee, Chem. – Eur. J., 2007, 13, 6130–6138 CrossRef PubMed
; C. B. Aakeröy, M. Baldrighi, J. Desper, P. Metrangolo and G. Resnati, Chem. – Eur. J., 2013, 19, 16240–16247 CrossRef PubMed
.
- C.-H. Weiss, D. Blaser, R. Boese, B. M. Doughan and M. Haley, Chem. Commun., 1997, 1703 RSC
; J. M. A. Harris, D. Philp, K. D. M. Harris and B. M. Kariuki, New J. Chem., 2000, 24, 1799 Search PubMed
.
- B. K. Saha and A. Nangia, Cryst. Growth Des., 2007, 7, 393 CAS
.
- J. Scheinbeim and E. Schempp, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32, 607–609 CrossRef
.
- M. Erdelyi, Chem. Soc. Rev., 2012, 41, 3547 RSC
; T. M. Beale, M. G. Chudzinski, M. G. Sarwar and M. S. Taylor, Chem. Soc. Rev., 2013, 42, 1667 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, details of the crystallographic work. CCDC 1021552–1021557, melting data, IR spectra, powder patterns and NMR spectra. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce02201d |
| This journal is © The Royal Society of Chemistry 2017 |