Coupled self-assembled monolayer for enhancement of Cu diffusion barrier and adhesion properties†
Abstract
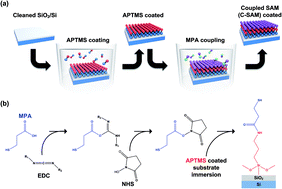
In this work, we have demonstrated chemically coupled (3-aminopropyl)trimethoxysilane (APTMS) and 3-mercaptopropionic acid (MPA) self-assembled monolayers (SAMs) to enhance the diffusion barrier properties against copper (Cu) as well as the adhesion properties towards SiO2 and Cu electrode. The coupled-SAM (C-SAM) can attach to both Cu and SiO2 strongly which is expected to enhance both the diffusion barrier and adhesion properties. A carbodiimide-mediated amidation process was used to link NH2 terminated APTMS to COOH terminated MPA. The resulting C-SAM shows a low root-mean-square roughness of 0.44 nm and a thickness of 2 nm. Time-dependent dielectric breakdown (TDDB) tests are used to evaluate APTMS and C-SAM for their ability to block Cu ion diffusion. The average time-to-failure (TTF) is enhanced over 4 times after the MPA attachment, and is even comparable to TaN barriers. Capacitance–voltage (C–V) measurements are also conducted to monitor Cu ion diffusion. Negligible change in the flatband voltage and C–V curve is observed during the constant voltage stress C–V measurement. Enhancement of the adhesion properties are measured using four-point bending tests and shows that the C-SAM has a 33% enhancement in the adhesion properties between SiO2 and Cu compared to APTMS. The C-SAM shows potential as an ultra-thin Cu diffusion barrier which also has good adhesion properties.

 Please wait while we load your content...
Please wait while we load your content...