Dynamic neighbouring participation of nitrogen lone pairs on the chromogenic behaviour of trans-bis(salicylaldiminato)Pt(ii) coordination platforms†
Abstract
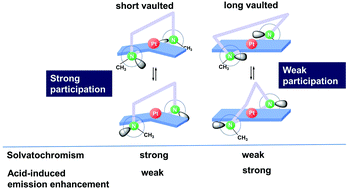
The participation of neighbouring nitrogen lone pairs in the chromogenic control of trans-bis(salicylaldiminato)Pt(II) platforms was examined, using newly designed Pt analogues bearing salicylaldehyde hydrazone ligands. A series of non-vaulted and vaulted Pt complexes (1–5) with salicylaldehyde hydrazones as trans-coordinated bidentate ligands were synthesized and characterized with regard to the chromogenic behaviour of the trans-bis(salicylaldiminato)Pt(II) coordination platforms. X-ray diffraction and 2D NMR data demonstrated that, in the case of the non-vaulted N-monomethyl complexes 1, there was significant participation of neighbouring N(2) lone pairs in the d–π conjugation of the trans-bis(salicylaldiminato)Pt(II) platforms owing to conformational fixation arising from intramolecular hydrogen bonding. In contrast, the lone pairs of the N,N-dimethyl analogues 2 made a much less significant contribution to the extension of the d–π conjugation, due to their high conformational mobility. Complexes 1–5 were found to have structure-dependent chromogenic properties in the solution state, such that the absorption spectra of the N-methyl, short-vaulted complexes 1 and 3 exhibited significant hypsochromic shifts relative to the N,N-dimethyl, long-vaulted analogues 2 and 5, which had spectra very similar to that of the trans-bis(salicylaldiminato)Pt(II) complex 6. The introduction of MeO groups at the 3- and 5 positions on the aromatic rings of 1 and 2 gave rise to significant hypsochromic and bathochromic shifts, respectively. In addition, νmax − ET(30) plots for various solvents revealed that complexes 1–5 exhibit negative solvatochromism, in which the data obtained in alcoholic solvents are hypsochromically separated from those in non-alcoholic solvents for 1 and 3, an effect that is not observed for 2 and 5. Complexes 1–5 also exhibit emission enhancement upon addition of excess CH3SO3H in dimethyl sulfoxide, and a significant effect of the linker on quantum yields (Φ77 K) was observed in the case of the vaulted complexes. Density functional theory calculations (B3LYP/6-31G*, LanL2DZ) determined that the structure dependence of the chromogenic behaviour of these non-vaulted and polymethylene-vaulted hydrazone complexes can be attributed to variations in the participation of neighbouring nitrogen lone pairs in the d–π conjugation on the trans-bis(salicylaldiminato)Pt(II) coordination platforms.

 Please wait while we load your content...
Please wait while we load your content...