Organocatalytic enantio- and diastereoselective synthesis of 3,5-disubstituted prolines†
Abstract
The asymmetric synthesis of substituted pyrrolidines has been accomplished using a novel organocatalytic cyclization reaction promoted by a Cinchona alkaloid based primary amine. The reaction proceeds smoothly yielding pyrrolidine-2,2-dicarboxylates after in situ diastereoselective reduction with high levels of enantioselection. Furthermore, these adducts could be easily transformed into N-protected disubstituted prolines through the base-promoted diastereoselective C → N alkoxycarbonyl transfer reaction.
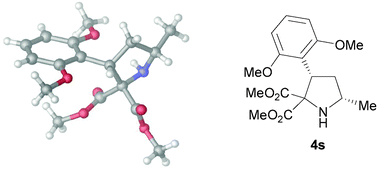

 Please wait while we load your content...
Please wait while we load your content...