Identification of intrinsic catalytic activity for electrochemical reduction of water molecules to generate hydrogen
Abstract
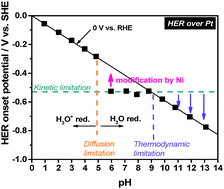
Insufficient hydronium ion activities at near-neutral pH and under unbuffered conditions induce diffusion-limited currents for hydrogen evolution, followed by a reaction with water molecules to generate hydrogen at elevated potentials. The observed constant current behaviors at near neutral pH reflect the intrinsic electrocatalytic reactivity of the metal electrodes for water reduction.

 Please wait while we load your content...
Please wait while we load your content...