A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for highly selective C2H2/CH4 and C2H2/CO2 gas separation at room temperature†
Abstract
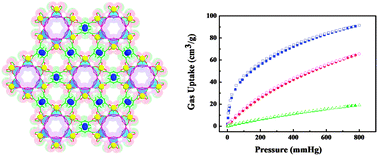
To immobilize both open metal and Lewis basic
- This article is part of the themed collection: 2013 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...