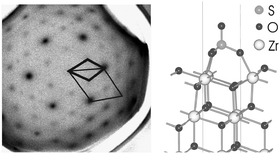
Single-crystalline sulfated c-ZrO2(111) films of the cubic (c) type have been prepared by reactive deposition of Zr onto Pt(111) in an O2 atmosphere and subsequent exposition to a SO3 atmosphere. The morphology, atomic structure, and composition have been examined by scanning tunneling microscopy, low-energy electron diffraction (LEED), Auger electron spectroscopy, and density functional theory (DFT) calculations. The clean c-ZrO2(111) films display a (2 × 2) surface structure. During SO3 exposure at room temperature, a clear (√3 ×
√3)R30° structure develops. At about 700 K, the SO3-induced (√3 ×
√3)R30° structure disappears and the bright (2 × 2) LEED pattern of the clean ZrO2 films reappears. The energies of plausible c-ZrO2(111)/SO3 structures have been examined by DFT. The (√3 ×
√3)R30° structure found in the experiments turned out to be the most stable one for temperatures below 700 K. At temperatures around 700 K, a disordered low coverage structure may exist, which can not be observed by conventional LEED. A comparison of cubic zirconia surfaces with the alternative tetragonal system yields similar results for the SO3 adsorption in the DFT calculations and shows that c-ZrO2 surfaces are good models for the industrial used tetragonal ZrO2 supports.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...