Synthesis of fused pyridines in the presence of thiamine hydrochloride as an efficient and reusable catalyst in aqueous conditions
Abstract
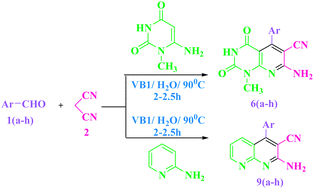
Efficient and straightforward synthesis of fused pyridine derivatives was achieved from electron-rich amino heterocycles and Knoevenagel products derived from aldehyde and malononitrile under aqueous media at 90 °C in the presence of thiamine hydrochloride as a reusable, green catalyst. The strategy in this protocol involves addition on an activated olefinic bond formed in situ by Knoevenagel condensation between an aromatic aldehyde and an active methylene compound. The Michael product on subsequent cyclo condensation yielded fused pyridine in high yield. It offers several advantages such as inexpensive, easily available and recyclable catalyst, simple operational procedure, excellent yield and use of aqueous medium that is considered to be relatively eco-friendly. Vitamin B1 was recovered and reused thrice.

 Please wait while we load your content...
Please wait while we load your content...