Selective reduction of SWCNTs – concepts and insights†
Abstract
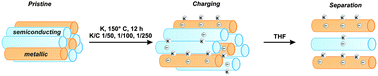
The charging of single-walled carbon nanotube (SWCNT) mixtures by reduction via alkali metal atoms is an established first step towards covalent SWCNT functionalization. In this combined density-functional theory and experimental study, we investigate this reduction with respect to differences occurring between tubes of different electronic type (metallic (m) and semiconducting (sc) tubes, respectively). We find that metals, specifically potassium, adsorb stronger to m- than sc-SWCNTs, which can be explained by the different band structures of both tube types. We investigate this trend in detail for a variety of different chiral SWCNTs, finding a potassium coverage dependent preference of m- over sc-SWCNTs, which is predicted to allow for a selective charging of metallic tubes for K/C ratios ≤ 1/200. This selective charging can be translated into the enrichment of m-SWCNTs during dispersion of SWCNT mixtures, since only reduced tubes are dissolved from the bulk material. The results for isolated tubes can be generalized to SWCNT bundle arrangements, which means that the theoretical predicted selective charging is transferable also to this more realistic description of the experimental systems. The theoretical findings regarding an electronic type selective charging of SWCNTs have been verified by an experimental study. By a combination of Raman and absorption/emission spectroscopic analysis, a preferential dispersion of charged metallic carbon nanotubes in THF as solvent was found for the predicted low potassium concentrations. Our results lead to the conclusion that previous m/sc selective reductive functionalization reactions cannot be explained on the basis of an electronic type selective charging step, as these reactions used much higher alkali metal concentrations.

 Please wait while we load your content...
Please wait while we load your content...