Lewis basic site (LBS)-functionalized zeolite-like supramolecular assemblies (ZSAs) with high CO2 uptake performance and highly selective CO2/CH4 separation†
Abstract
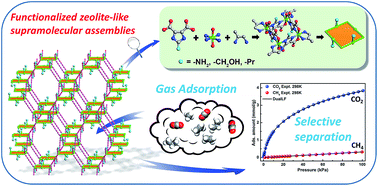
Herein, three functional isoreticular zeolite-like supramolecular assemblies (ZSAs), [Co4(NH2-ImDC)4(1,2-PDA)4]·8H2O (ZSA-7), [Co4(CH2OH-ImDC)4(1,2-PDA)4]·8H2O (ZSA-8), and [Co4(Pr-ImDC)4(1,2-PDA)4]·9H2O (ZSA-9), NH2-ImDC = 2-amino-4,5-imidazoledicarboxylic acid, CH2OH-ImDC = 2-hydroxymethyl-4,5-imidazoledicarboxylic acid, Pr-ImDC = 2-propyl-4,5-imidazoledicarboxylic acid, 1,2-PDA = 1,2-propanediamine, were hydrothermally synthesized and structurally characterized. All three compounds are isoreticular to ZSA-1, with the modification of different functional groups of –NH2, –CH2OH, and –Pr in imidazole dicarboxylate ligands. ZSA-7 and ZSA-8 display high CO2 adsorption abilities (109.8 and 114.0 cm3 g−1 at 273 K, respectively, under 1 bar) and outstanding natural gas selectivity separation, especially for CO2 over CH4 (39.8 and 35.7 for CO2/CH4 = 0.5/0.5 and 76.0 and 51.6 for CO2/CH4 = 0.05/0.95, respectively, under 1 bar at 298 K). In contrast, ZSA-9 is used for comparison to illustrate the effect of the introduction of Lewis basic sites (LBSs). Thus, all three ZSAs are promising materials for gas adsorption and purification applications.


 Please wait while we load your content...
Please wait while we load your content...