Sterically controlled diastereoselectivity in thio-Staudinger cycloadditions of alkyl/alkenyl/aryl-substituted thioketenes†
Abstract
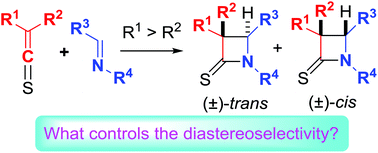
The [2 + 2] cycloadditions of thioketenes and imines are named as thio-Staudinger cycloadditions. The diastereoselectivity in thio-Staudinger cycloaddtions of alkyl/alkenyl/aryl-substituted thioketenes is rationalized. The steric effects of the thioketenes play an extremely important role in deciding the diastereoselectivity (cis/trans selectivity) through controlling exo- and endo-attack and subsequent ring closure. The conclusion is further supported by our additional experimental and calculational results. The isomerization of the iminium moiety in zwitterionic intermediates generated from the thioketenes and linear imines also affects the diastereoselectivity. The electronic effect of imine substituents slightly impacts the diastereoselectivity, while epimerization of cis-β-thiolactams to trans-diastereomers is a significant factor in the thio-Staudinger cycloadditions of mono-substituted thioketenes under basic conditions.


 Please wait while we load your content...
Please wait while we load your content...