Fluorine substitution enhances the self-assembling ability of hydrogelators†
Abstract
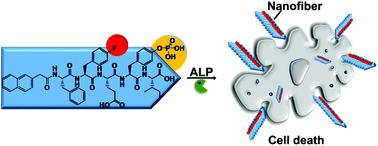
When supramolecular hydrogels are applied as tissue culture scaffolds, their mechanical strength and biocompatibility are the two most important factors that must be considered. However, systematic studies on the structure–mechanical property (or structure–cytotoxicity) relationship of hydrogels are rare. Herein, we rationally designed three hydrogelators and their corresponding phosphate precursors, and systematically studied their self-assembling ability and cytotoxicity. The results indicated that fluorine substitution, but not trifluoromethyl substitution with more fluorine atoms, to the phenylalanine motif enhanced the self-assembling ability and cytotoxicity of the hydrogelators (or precursors). We envision that our preliminary study of hydrogelator fluorination would provide a strategy for the development of supramolecular hydrogels for wider biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...