Efficient and recyclable Ir(i)-catalysts with the involvement of π-acceptor phosphines for N-alkylation of aryl amines with alcohols†
Abstract
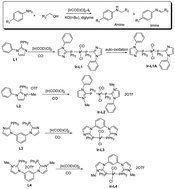
Mono-phosphines (L1 and L2) and diphosphines (L3 and L4) with typical π-acceptor character were prepared and applied in Ir(I)-catalyzed N-alkylation of amines with alcohols. It was found that the π-acceptor character of the applied phosphines is closely correlated to the catalytic efficiency of the [Ir(COD)Cl]2 complex for this green reaction. Compared to PPh3 as the typical σ-donor (i.e. with poor π-acceptor character), L1–L4 all resulted in the efficient N-alkylation of aniline to benzyl-aniline. While L1–L4 coordinated to the Ir(I)-catalyst, the consolidated Ir–P linkages due to π-backdonation could well protect the Ir-catalyst against deactivation, giving rise to the N-alkylation of aniline. On the other hand, the stabilized Ir-catalyst with the involvement of L2 could be recycled successfully for at least 4 runs in the ionic liquid of [MePh3P]Br without the detectable leaching of Ir and P elements in the organic products.


 Please wait while we load your content...
Please wait while we load your content...