NPY binds with heme to form a NPY–heme complex: enhancing peroxidase activity in free heme and promoting NPY nitration and inactivation
Abstract
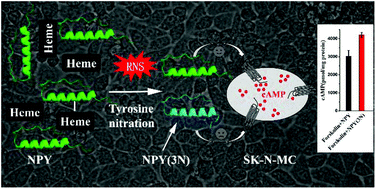
Neuropeptide Y (NPY) is a member of the pancreatic peptide family of neuropeptides that play a crucial role in numerous central and peripheral nervous system responses. Recently, it has been shown that NPY protects cells against neurotoxic damage from β-amyloid peptides (Aβ) in Alzheimer's disease (AD). Heme is a common factor linking several metabolic perturbations in AD and altered heme metabolism has been shown to be related to the pathologies of AD. Thus, heme may have a chance to act on NPY and potentially counteract its function. To explore this, UV-visible spectroscopy, fluorescence spectroscopy and differential pulse voltammetry (DPV) were used to demonstrate that NPY can bind with heme to form a NPY–heme complex and the binding enhances the peroxidase activity of heme. Dot blotting results indicate that NPY is easily nitrated upon binding with heme when H2O2 and NO2− are present. Furthermore, LC-MS/MS results confirm that tyrosine36 (Tyr36), an important amino acid residue of NPY in binding and activating neuropeptide receptors, can be nitrated during the nitration process. Thereafter, we used mutant peptide NPY(3N) (Tyr36 replaced by 3-nitrotyrosine) to investigate the impact of nitration on the structure and bioactivity of the peptide. Our results show that Tyr36 nitration destabilizes the α-helix conformation of the peptide, and counteracts NPY-induced inhibition of cAMP accumulation in SK-N-MC cells. Collectively, these data imply that the self-association of NPY with heme potentially induces tyrosine nitration, destroys the active monomeric conformation of the peptide and thereby counteracts its bioactivity.


 Please wait while we load your content...
Please wait while we load your content...