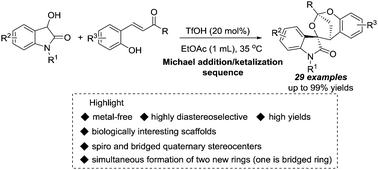
Metal-free diastereoselective construction of bridged ketal spirooxindoles via a Michael addition-inspired sequence†
Abstract
A TfOH-catalyzed highly diastereoselective Michael addition/ketalization sequence of 3-hydroxyoxindoles and ortho-hydroxychalcones was developed, leading to biologically important bridged ketal spirooxindoles in moderate to excellent yields. Moreover, some chemical transformations were carried out to further extend the structural complexity and diversity.


 Please wait while we load your content...
Please wait while we load your content...