Cobaloxime-catalyzed hydration of terminal alkynes without acidic promoters†
Abstract
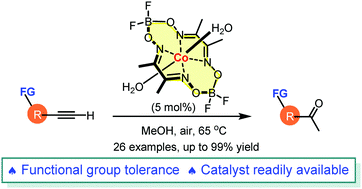
Cobaloxime (Co(dmgBF2)2·2H2O), an inexpensive first-row transition-metal complex, catalyzed hydration of terminal alkynes gave the corresponding methyl ketones in good to excellent yields under neutral conditions (additional protic acids and silver salts are not required). A wide range of functional groups, such as allyl ether, benzyl ethers, carboxylic esters, imides, amides, nitro, and halogens, were tolerated. The mild reaction conditions together with the inexpensive feature and easy availability of the catalyst well address the current challenges in the field of alkyne hydration.


 Please wait while we load your content...
Please wait while we load your content...