Understanding the stability of MnPO4†
Abstract
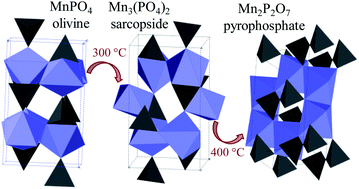
We have revealed the critical role of carbon coating in the stability and thermal behaviour of olivine MnPO4 obtained by chemical delithiation of LiMnPO4. (Li)MnPO4 samples with various particle sizes and carbon contents were studied. Carbon-free LiMnPO4 obtained by solid state synthesis in O2 becomes amorphous upon delithiation. Small amounts of carbon (0.3 wt%) help to stabilize the olivine structure, so that completely delithiated crystalline olivine MnPO4 can be obtained. Larger amount of carbon (2 wt%) prevents full delithiation. Heating in air, O2, or N2 results in structural disorder (<300 °C), formation of an intermediate sarcopside Mn3(PO4)2 phase (350–450 °C), and complete decomposition to Mn2P2O7 on extended heating at 400 °C. Carbon coating protects MnPO4 from reacting with environmental water, which is detrimental to its structural stability.

 Please wait while we load your content...
Please wait while we load your content...